Laser speckle contrast imaging (LSCI) is a technique used for imaging blood flow and tissue motion in biological samples. It leverages the speckle pattern produced when coherent light, such as that from a laser, interacts with a rough surface or inhomogeneous medium. Laser light reflecting off or passing through a rough or moving surface creates a random interference pattern known as a speckle pattern. The contrast of the speckle pattern is related to the speed and nature of motion in the sample. Areas with faster motion exhibit lower speckle contrast, while static or slowly moving areas have higher contrast. In biological tissues, red blood cells moving within vessels cause changes in the speckle pattern. LSCI can be used to monitor blood flow in tissues, providing valuable information in medical and research applications.
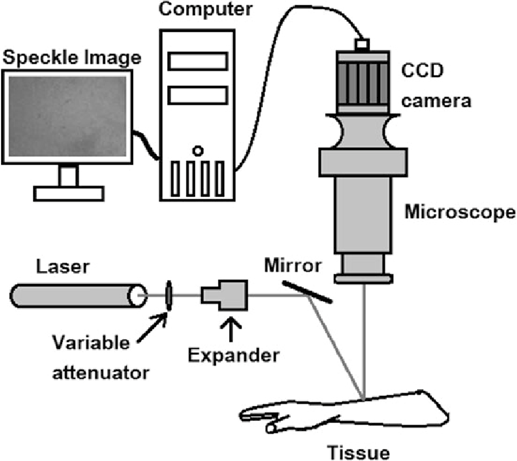
Figure 1 A typical LSCI system
Figure 1 shows a typical setup of an LSCI system. A coherent laser source, often in the red or near-infrared wavelength range, generates the laser beam used to illuminate the sample. A beam expander may be used to widen the laser beam, ensuring uniform illumination over the sample. Lenses and optical components are used to focus the laser beam onto the sample and collect the backscattered or transmitted light. A high-speed camera or image sensor captures the speckle pattern formed on the sample’s surface. A computer or dedicated data processing unit processes the captured images to calculate speckle contrast. This involves analyzing pixel intensity fluctuations over time. Software tools visualize the speckle contrast images and may provide quantitative information about blood flow or tissue motion.
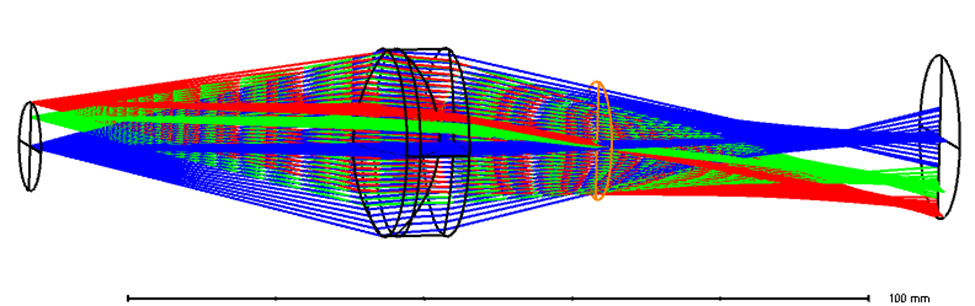
Figure 2 LSCI detection path modeled in Zemax sequential mode
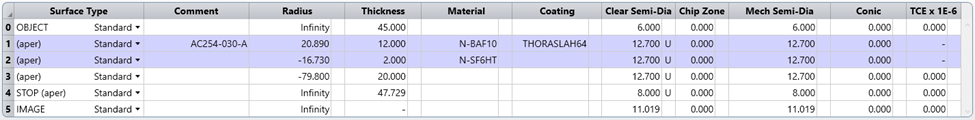
Figure 3 Lens data of the detection path
Figure 2 shows a simplified detection path of an LSCI system. An off-the-shelf lens from Thorlabs with back focal length of 30 mm and aperture of 1 inch represents the detection optical projector. The region of interest (ROI) is about 25 mmx 25 mm, with diagonal around 18mm. Ray colors represent fields, which are represented with object height of 0, 4 mm and 6 mm. In this case the object mimics a camera sensor. The conjugated image represents the specimen of LSCI image.
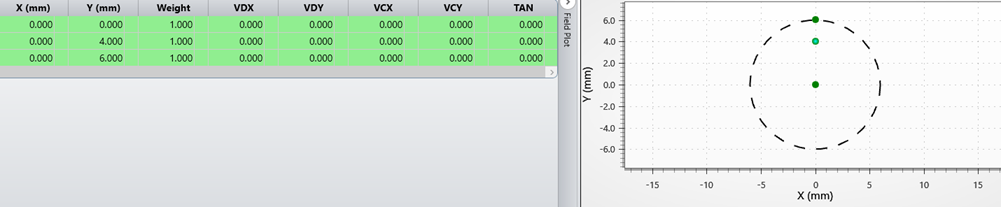
Figure 4 Field setting of LSCI detection optics
The coherent light generate speckle image at the surface of specimen, the speckle image is shown below.

Figure 5 Speckle image generated by coherent light source
Accurate estimation of the speckle contrast from the spatial statistics of the speckle pattern generally assumes that the speckle intensity distribution follows a negative exponential probability distribution function. We always mentioned that one requirement for this is that the speckle pattern be fully evolved, i.e., that the received light has an effectively uniform phase distribution. Additionally, care must be taken in spatial sampling of the speckle pattern. Specifically, the speckle size relative to the camera pixel size must be considered as well as the number of pixels that are used to estimate the speckle contrast. the speckle pattern is imaged onto a camera in which case the minimum speckle size will be given by:
![]()
where l is the wavelength of light, M is the magnification of the imaging system, and f /# is the F number of the system. The Nyquist sampling criteria must be satisfied by having the minimum speckle size be two times larger than the camera pixel size, i.e.
![]()
to obtain a negative exponential distribution.
In terms of the conditions above, Zemax is able to help developers in identifying magnification M and f number to satisfying Nyquist sampling. The Analyze – System Data tab offers magnification (1.89) and F number (1.198) calculations below. Note that, magnification M should take reciprocal of the Zemax number (1/1.89) to match the Nyquist sampling rule.
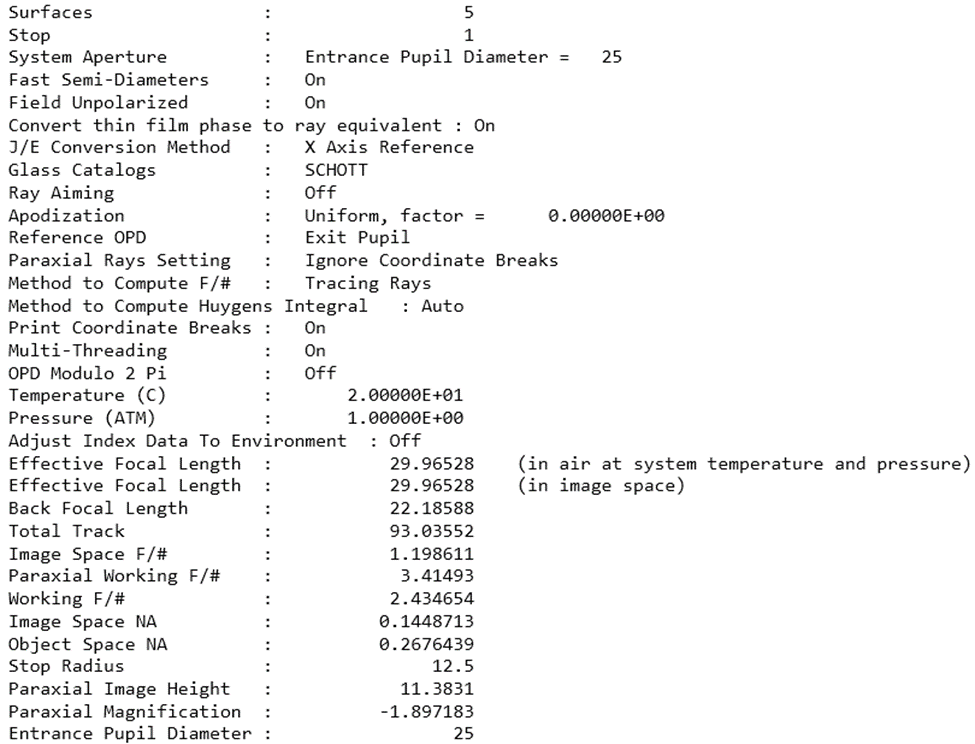
Figure 6 System data of the imaging system for Nyquist sampling criteria analysis
If the M and F number shown in Figure 6 doesn’t match the Nyquist sampling, the geometrical optical parameter in the detection optics needs to be adjusted. The stop size, i.e. object 4 in Figure 3. This size can be changed by a physical adjustable diaphragm in the system. The location of the diaphragm can also contribute to satisfying the Nyquist sampling criteria.
It’s important to note that the specific components and configurations of the optical path may vary depending on the design of the LSCI system and its intended application. Overall, the goal is to capture dynamic information related to blood flow or other motion within biological tissues by exploiting the speckle pattern produced by the interaction of laser light with scattering particles.

