Metalloids are unique natural elements that possess characteristics of both metals and nonmetals. Their distinctive properties make metalloids irreplaceable in various scenarios. This article explores this group of elements, discussing their properties, applications, and positions in the periodic table. We will also provide a brief overview of each commonly recognised metalloid.
- Metalloids exhibit properties that are a blend of both metals and nonmetals, making them versatile for a wide range of general and specialised applications.
- They occupy a zigzag line in the periodic table. Metals lie on one side of this diagonal region and nonmetals lie on the other. This arrangement also indicates their hybrid properties.
- Metalloids hold significant importance in technology, particularly in the manufacturing of semiconductors. Semiconductors are essential components in computers, electronics and solar panels.
- Some metalloids, such as tellurium and antimony, hold significant economic and strategic importance due to their scarcity, which can severely impact defense, energy production and automotive industries.
What Are Metalloids?
Metalloids are distinctive elements that possess properties intermediate between metals and nonmetals. These intermediate properties include electronegativity, density, ionisation energy, strength, thermal conductivity, melting point and electrical conductivity.
The specific set of physical, chemical and mechanical properties found in metalloids makes them ideal for specialised applications, such as semiconductors and solar power generation. Additionally, they are widely utilised in various general applications, including medicines, herbicides and insecticides.
One of the reasons for the intermediate properties of metalloids is the number of valence electrons in their outer shell. Metals typically possess one to three electrons in their outer shell, while nonmetals have four to seven. Metalloids contain three to six electrons in their valence shell, enabling them to form intermetallic compounds with metals (ionic bonds) and covalent bonds with nonmetals.
Some sources identify six metalloid elements, while others recognise as many as eight. The commonly recognised elements in the metalloid group include boron (B), arsenic (As), silicon (Si), antimony (Sb), polonium (Po), tellurium (Te), germanium (Ge), and astatine (At).
Metalloids on the Periodic Table
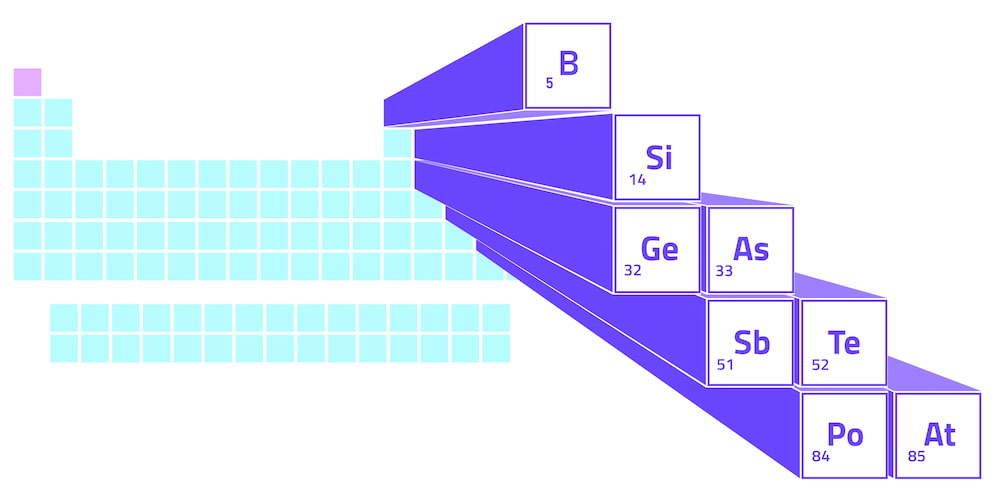
Metalloids form a zigzag line in the p-block of the periodic table, which is the raised section on the right side of the table. This zigzag line begins at boron in group 13, moves diagonally to silicon, and then continues in a step-like fashion to astatine in group 17. Groups refer to the numbering of the columns in the periodic table.
On the left side of the zigzag metalloid line, we find metals (with the exception of hydrogen). On the right side, we have nonmetals. Therefore, the metalloids create a dividing line between metals and nonmetals in the periodic table.
General Properties of Metalloids
Metalloids exhibit a unique combination of properties that are not present in other elements. Some of these properties include:
Variable Electrical Conductivity
The electrical conductivity of metalloids is intermediate, falling between that of metals and nonmetals. This is attributed to their electronic band structures, which resemble those of semimetals or semiconductors.
However, we can enhance their conductivity through doping. This process allows the creation of essential electronic components, such as diodes, transistors and integrated circuits, which are fundamental to modern computing.
Appearance
Metalloids have a metallic appearance and are solid at room temperature. Their shiny, reflective surfaces often lead to their misidentification as metals. This metallic lustre is also the reason behind the origin of their name; metalloid means metal-like in Latin/Greek.
Thermal Conductivity
Metalloids exhibit moderate thermal conductivity, which is higher than that of non-metals but lower than that of metals. Even among the eight metalloids, thermal conductivity varies significantly. Some metalloids are effective heat conductors, while others function as nearly perfect insulators. This property allows for the use of certain metalloids in thermoelectric devices.
Brittleness
Unlike metals, metalloids do not have the physical properties of ductility and malleability. They break easily and lack the strength required for structural applications.
Chemical Reactivity
Metalloids are fairly reactive with metals and nonmetals. They are common in metal alloys as additives to enhance the properties of transition metals, providing benefits such as increased strength and improved corrosion resistance.
With non-metals, metalloids form covalent bonds by sharing electrons. Their reactions with halogens form compounds such as boron trifluoride, antimony trioxide and silicon tetrahalide.
Common Metalloids and Their Applications
All metalloids share certain common properties; however, unlike other groups of elements such as halogens, alkali metals, or alkaline earth metals, each metalloid also possesses unique characteristics. This diversity allows for the utilisation of metalloids in a wide range of applications.
Boron (B)
Boron is a black, lustrous metalloid that constitutes approximately 0.001% of the Earth’s crust by weight. It is the hardest of all metalloids and exhibits excellent heat resistance. Its Mohs hardness value is 9.3, compared to 10 for diamond, which is the hardest known material.
Boron is a versatile element with a range of applications. One of these is to produce borosilicate glass, a special glass with high heat and chemical resistance. It is also used to produce flame retardants, cosmetics, insecticides and detergents.
Nickel-boron alloys are used as master alloys to form alloys such as nickel-based superalloys, special steels and welding alloys.
Boron is also instrumental in silicon doping. Doping enables us to produce silicon-based semiconductors that are indispensable to modern computing.
Silicon (Si)
Silicon is the most well-known metalloid and the second most abundant element on Earth, following oxygen. It constitutes approximately 27% of the Earth’s crust by weight.
It is generally inert at ambient temperatures, however, its reactivity increases with temperature. It combines with oxygen to form silica (silicon dioxide), which is found in most clays, rocks, sands, and soils. It combines with most metals and metallic alloys, imparting properties such as fluidity, corrosion resistance, strength and heat resistance.
Silica and pure silicon are essential materials in modern computing. Silica is used to produce capacitors that store electrical energy in circuits. Silicon, which is used to manufacture critical electronic chip components, allows us to create smaller, faster and more powerful electronic devices.
Germanium (Ge)
Directly below silicon in the periodic table, we have the metalloid germanium with an atomic number of 32.
Germanium is more abundant than other metalloids, such as arsenic and antimony. However, its high reactivity with other elements means that it is not found in its elemental form in nature. At room temperature, germanium has a hard and brittle structure and does not react with air. However, as the temperature increases, its reactivity also increases, leading to the formation of oxides at approximately 600-700 degrees Celsius (1000-1100 degrees Fahrenheit).
Germanium is also used in semiconductor applications, although to a lesser extent than silicon. Additionally, it has applications in medicine and coin production.
Arsenic (As)
The chemical element arsenic, which belongs to group 15 and has an atomic number of 33, is a metalloid known for its toxic nature. At room temperature, arsenic is stable and does not react with air. However, in the presence of moisture, it oxidises to form a golden-bronze tarnish that eventually turns black.
Long-term exposure to arsenic through food and water can lead to serious illnesses, including cancer. However, it is worth noting that arsenic is an essential trace element for some organisms. It plays a direct and indirect role in the production of both beneficial and harmful biological agents.
Arsenic is also used in other sectors such as glass manufacturing, semiconductor production, agricultural chemicals and mining.
Antimony (Sb)
Antimony, with the atomic number 51, is found in group 15 of the periodic table, directly below arsenic. It possesses a hard and brittle structure, characterised by a silvery appearance.
Most antimony is extracted from its sulfide mineral, stibnite, with China, Russia, Bolivia, and Kyrgyzstan holding the majority of antimony reserves. Besides, China is the largest producer of antimony in the world.
Antimony has traditionally been used in medicinal and cosmetic applications. Over time, it has also become valuable in the production of flame-retardant materials, paints, batteries, glass, pottery, optical storage media and semiconductor devices such as diodes and infrared detectors.
For Europe and the United States, antimony is a critical element, as 100% of it is imported. A disruption in supply could severely impact essential industries such as automotive, construction, and defence.
Tellurium (Te)
Tellurium is a rare, silvery-white metalloid that was first discovered in gold mines as gold telluride (aka calaverite). With an abundance of 1 part per billion (1 microgram per kilogram), tellurium is as rare as platinum.
No large-scale applications of tellurium have been identified to date. In industry, tellurium is primarily used as an alloying element and in the production of solar panels and thermoelectric devices.
Polonium (Po)
Polonium is a radioactive metal that is sometimes classified as a metalloid. It has 42 isotopes, none of which are stable and all of which are radioactive. The half-life of Polonium isotopes ranges from 115 nanoseconds (billionth of a second) for Po-205m4 to 124 years for Po-209.
Polonium has several applications, all of which use its radioactive properties. Some of these are:
- As a thermoelectric generator for spaceflight
- As an antistatic device to eliminate static charge
- As a neutron source in combination with beryllium
Astatine (At)
Astatine is generally classified as a metalloid, however, some classifications categorise it as a nonmetal. It is the second-to-last element in the halogen group, preceded by fluorine, chlorine, bromine and iodine, and it exhibits several common properties with these elements.
Astatine is highly radioactive, and its properties have yet to be studied in detail, as it has never been synthesised in sufficient quantities for thorough analysis. Besides, it sublimates so quickly that you can lose half of it within an hour.
Astatine has limited applications due to its radioactivity and short half-life. Nevertheless, it has been utilised in research, primarily as a radioactive tracer and in treating thyroid cancer.
Conclusion
Metalloids are an important group of elements that bridge the gap between metals and nonmetals. Their combination of metal and nonmetal properties enables their use in very diverse industries, from agriculture to optical storage and optoelectronics.
Their semiconducting properties, in particular, have significantly advanced technology and modern computing. Some metalloids, such as arsenic, are toxic, however, their controlled use offers unparalleled benefits across various sectors.
Due to the above reasons, the demand for metalloids is expected to grow. However, the scarcity of certain metalloids has rendered them critical elements. For example, antimony production is declining as existing sources are depleting rapidly, with no new substitutes being discovered.
This has made it increasingly important to identify sustainable sources for metalloids. Efforts are underway to research, conserve, and recycle metalloids.

