Many manufacturing processes leave residues on metal surfaces that must be cleaned before further processing or prior to putting the metal parts into use. These surface impurities can exist in the form of metal oxides, stains, rust and other contaminants.
Processes such as grinding, abrasive blasting, or wire brushing are often used to achieve these objectives. However, these processes can be less accurate and may either remove excessive or insufficient amounts of metal at times.
In this article, we shall explore an alternative, a more reliable surface finishing process known as pickling, which is commonly used to remove scale and rust from metal surfaces. Let’s begin.
What Is Metal Pickling?
Metal pickling is the process of cleaning contaminated metal surfaces with specific chemicals before further processing or coating. It is most commonly performed after hot working processes since they tend to leave a discolored oxide layer on the surface. To remove this scale, the workpiece is immersed in acid baths. Thus, prior to cold rolling operations, hot rolled steel usually goes through a pickling line to remove surface scale.
Different metals and applications use various acids but the most common ones are hydrochloric acid and sulphuric acid. Since the chemicals used are commonly known as pickle liquor, the process came to be known as pickling. It is sometimes referred to as acid washing if descaling is not needed.
The metal parts are exposed to the pickling liquids through immersion bath, spray, or application with a brush. In the case of pipes that need to be pickled internally, the pickling liquid is pumped through them.
Pickling is most often performed on ferrous metals, noble metals, copper and aluminium alloys. Pickled metal has various benefits such as an attractive surface, increased durability, increased corrosion resistance, and removal of heat-affected zones.
Pickling Process – How Is Steel Pickled?
The pickling process is pretty straightforward and it can be divided into six simple stages:
Pickling of Hot Rolled Steel Coil
Pre-cleaning
The precleaning step refers to cleaning the material using degreasing or caustic solutions. Surface impurities such as loose oil, dirt and any other contaminants must be removed during this step.
Pickling solution preparation
We typically use diluted hydrochloric or sulfuric acid for the pickling process. The concentration and temperature vary for different metals and the level of pickling required. You can find the ideal concentrations by referring to the project requirements or manufacturer’s guidelines.
The solution must be prepared with extreme caution. Acid should always be added to the water, and not the other way around, to prevent splattering of concentrated acid.
Pickling
In this stage, we immerse the carbon steel in a bath of hydrochloric or sulphuric acid if the carbon content is less than 6%. If the carbon content is higher, the steel must first be pickled with hydrofluoric, nitric or phosphoric acid.
The material is submerged fully in the solution for a predetermined amount of time. The duration depends on the level of contamination and the type of metal. The range can be from a few minutes to a few hours. The solution must be stirred from time to time.
A small amount of material (1 to 3% of steel mass) is removed from the surface by chemical reactions, freeing the material of iron oxides. The steel is removed from the solution once sufficient cleaning levels are achieved.
The left-over pickling liquor, now contaminated with rust flakes and iron oxide dust, is called pickling sludge. It is possible to recover some of the acid and ferric oxide through regeneration but the rest must be disposed of properly as it is classified as hazardous waste.
Rinsing
The steel surface must be cleaned of all the acid by rinsing it with clean water. All traces of the pickling solution must be removed before proceeding with further processing.
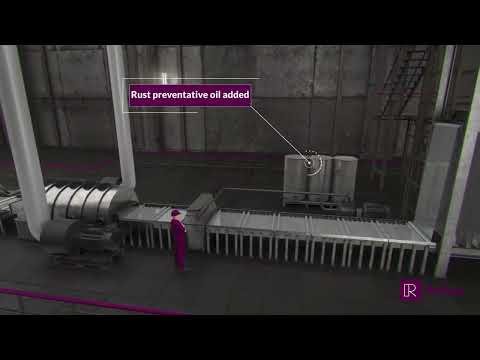
Neutralisation and rust inhibitor application
In some cases, the part is neutralised using an alkali solution to thoroughly cleanse the surface and remove any traces of the pickling agent. A commercial neutraliser or baking soda, is suitable for this purpose. Alkaline solutions are also supplemented with rust inhibitors to enhance the corrosion-resistant properties of the part.
Drying
After rinsing and neutralising, the part is dried before considering the process complete. Once this step is completed, the part is ready for additional processing or coating, as required.
Acids Used for Pickling
The most common acids used for steel pickling are hydrochloric acid and sulfuric acid. These chemicals also work for a variety of metals other than steel.
Hydrochloric acid is more common now, but previously, it used to be sulphuric acid. Sulphuric acid is more affordable than hydrochloric acid, but it is unsuitable for fast steelmaking lines due to its requirements for longer contact durations. Automatic steel mills can produce hot rolled steel at rates of up to 243 m/min, which necessitates fast pickling times.
Hydrochloric acid is also more efficient at removing scale formation and has reduced heating costs since the process can occur at room temperature. It also has less hydrogen penetration through diffusion and reduced depositions of iron salt on a pickled part. However, hydrochloric acid can be difficult to recover and it also starts to fume at slightly elevated temperatures. It is also more corrosive and harder to dispose of than sulphuric acid.
Sulphuric acid, besides being more affordable, has the advantage of being able to manipulate the pickling rate by adjusting the process temperature. Increased temperatures allow for lower acid concentrations to pickle more effectively. Iron sulfate formed during pickling with sulphuric acid is easier to recover, and the acid can be renewed more frequently.
But sulphuric acid attacks the base metal more aggressively. It also promotes deeper diffusion of hydrogen. The pickling residues are also more adhesive and the process almost always requires heating.
Carbon steels with a carbon content greater than 6% (cast iron) are initially pickled using phosphoric acid, nitric acid, and hydrofluoric acid before being treated with a hydrochloric or sulphuric acid solution. Nitric and hydrofluoric acids are also suitable for pickling corrosion-resistant and acid-resistant chromium-nickel steels.
Phosphoric acid is the best option for removing a thin film of iron oxide or iron scale. The process is also relatively expensive. However, the use of phosphoric acid leads to the formation of a thin layer of iron phosphate, which immediately passivates the metal.
Copper alloys are typically pickled using dilute sulphuric acid, while brass is best pickled with a mixture of concentrated nitric and sulphuric acid, along with soot and sodium chloride.
Benefits of Pickling
Pickling of materials is done for the following advantages:
- The materials are cleaned of all surface impurities, such as rust and scale. The end result is a smooth and clean surface finish.
- The process removes heat tint from previous operations. The final product has a uniform colour that is achieved through very few finishing processes.
- It improves the corrosion resistance of the material, as is the case with stainless steel.
- Less abrasion of the metal surface compared to the mechanical method. Chances of particle embedment are also eliminated.
- Enhances the surface’s appearance. Jewellery is often pickled to remove the unattractive copper oxide layer from the surface.
- Pickling is a simple, affordable and relatively easy process.
- We can recover valuable products from the pickling sludge by sending the waste mill scale to a sintering plant, where it can be transformed into a solid steel mass.
Limitations of Pickling
- It is difficult to create consistent results as the acid becomes weaker over time. The amount of material removed varies over time as the acid bath degrades.
- The pickling sludge is a hazardous waste. Strict EPA regulations around this waste material limit its use to some extent.
- The handling of hazardous pickling chemicals is a significant safety concern in the pickling process.
- In some cases, hydrogen diffusion can result in hydrogen embrittlement, causing steel to become brittle and weak, and negatively impacting the material’s physical properties.