Corrosion is a destructive phenomenon wherein the surface of the metal deteriorates from chemical or electrochemical reactions. Its pervasive nature impacts virtually all industries – infrastructure, electronics and the automotive industry, to name a few.
Let’s delve into the nature of corrosion, the types of corrosion and how to combat their effects.
What Is Metal Corrosion?
Metal corrosion occurs when the metal surface reacts with a corrosive environment or is subjected to other unfavourable conditions that cause the surface to corrode. Oxidation or rust formation occurs on the exposed surface of the metal and causes the material to weaken over time, leading to structural damage. Corrosion is not only specific to metals, it can occur in other materials, such as polymers and ceramics, for example. However, for these materials, the term degradation is more common.
Corrosion is a natural process that converts a refined metal to a more stable metal oxide. An electron exchange occurs between the metal and the environment, with the metal losing electrons in the process. Metals corrode naturally over time, but the type of environment that the material is subjected to can accelerate the corrosion process. Stable metals, such as gold and platinum, are less likely to corrode due to their stable chemical nature. Alloys that contain certain elements, such as iron, are more prone to rusting when exposed to air and moisture.
Localised corrosion forms cracks and pits, however, corrosion may span over the entire surface of a metal. Corrosion in metal surfaces is influenced by a variety of factors, such as acidity levels in the environment, temperature, mechanical stress, and humidity. These factors may either induce accelerated deterioration of the metal surface or rapidly speed up the corrosion process, ultimately leading to failure.
Protective coatings, galvanisation, and heat treatment are general methods for reducing the risk of corrosion. However, corrosion may still occur under specific conditions due to its complex nature.
Types of Corrosion
There are several types of corrosion that are all influenced by a variety of mechanisms and conditions. Let’s explore each type and understand how they occur.
General Corrosion
General or uniform corrosion is the most common type of corrosion as it occurs across the surface of a metal. This is commonly caused by the absence of a protective coating, leaving the metal exposed to corrosive agents. Electrochemical and chemical reactions occur which cause the metal to dissolve, allowing it to thin out as it forms oxides.
Continuous exposure to these corroding agents will eventually dissolve the whole metal structure. Examples of metals affected by uniform corrosion are aluminium, iron, lead, steel, and zinc. This type of corrosion is predictable and detectable to the naked eye as it is visible in the form of rust over the entire surface.
Pitting Corrosion

Pitting corrosion is an unpredictable type of localised corrosion wherein rust pits form at the metal surface. These pits grow into cavities or holes, penetrating the surface in a downward direction. This type of corrosion is caused by structural defects, poor coating application, non-uniformities, moisture, or damage to the protective oxide layer of the metal. Early stages of pitting corrosion have pit diameters of ≤20 µm.
Pitting corrosion is an insidious type of corrosion since only a small amount of material on the surface is lost while the deep metal structure is damaged. Failure due to pitting can be sudden and is often devastating. Pitting can be observed in aluminium, nickel alloys, and steel. Polished metal surfaces are more resistant to pitting due to their more uniform surface area.
Crevice Corrosion
Crevice corrosion is another type of local corrosion that occurs at the crevices or confined areas, usually between two metals (commonly found in assemblies), a metal and nonmetal, or from debris. These restricted areas usually allow a buildup of corrosive fluids while only having a limited supply of oxygen which prohibits shielding of the material. The imbalance in the pH level turns the fluid within the crevices acidic, which in turn breaks down the passive oxide layer, leaving the surface vulnerable to corrosion attack.
The risk for crevice corrosion can be reduced through a better assembly or a joint design where gaps are eliminated between welds and joints. Protection from moisture or fluids which promote an electrolytic environment may further reduce the risk of this form of corrosion.
Galvanic Corrosion
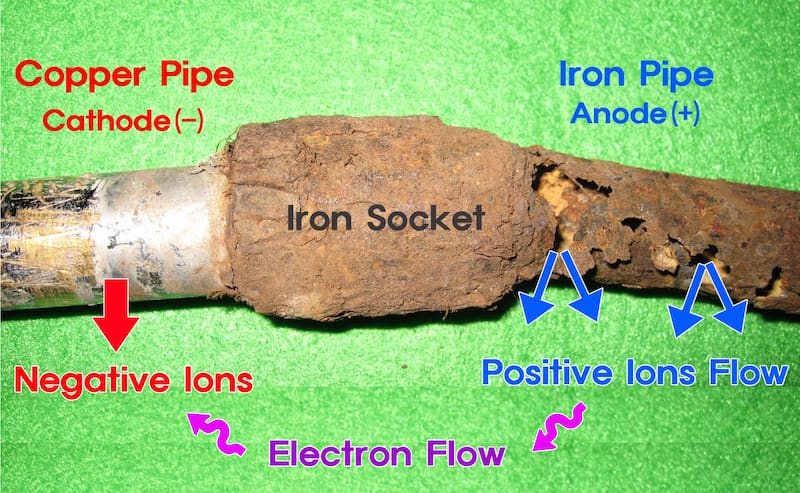
Galvanic corrosion or bimetallic corrosion is a type of corrosion wherein two dissimilar metals in physical and/or electrical contact are subjected to an electrolytic environment. The active metal (anode) undergoes corrosion at a faster rate than the other metal (cathode) which is more stable. It can also occur in a metal that is exposed to an electrolyte with varying degrees of concentration.
Galvanic corrosion is common in sea vessels where saltwater acts as an electrolyte between the two dissimilar metals. It can also occur in toxic environments, such as in handling molten metal or in chemical laboratories.
A sacrificial anode or galvanic anode that is more active than the metal workpiece is used to prevent/lessen galvanic corrosion. These consumable anodes prevent the main metals from being oxidised by supplying their electrons, corroding the consumable anode instead.
Fretting Corrosion
Fretting corrosion occurs when two metals in contact undergo small movements caused by slips and vibrations. These oscillations lead to fretting that removes the protective oxide film and allows surface asperities on freshly exposed metals to stick to one another. This connection is subsequently broken again by the vibrations, causing the build-up of wear debris.
This debris and the freshly exposed metal surfaces are prone to oxidation. Since the particles cannot escape the contact, they initiate further abrasive wear and subsequent oxidation, and the process continues with elevated wear volumes.
Reactive soft metals have lower corrosion resistance since the oxide layer is easy to remove under cyclic loading and material transfer takes place.
Intergranular Corrosion
Intergranular corrosion is a form of corrosion that occurs at or adjacent to the metal’s grain boundaries. Grain boundaries act as an interface between grains in the material and are considered imperfections in the material’s crystal structure. These grain boundaries result from uneven growth or impurities present as the metal alloy crystallises. Each grain can vary from 1 µm to 1 mm in size.
Intergranular corrosion or intergranular attack exhibits corrosion when the grain boundaries have a difference in reactivity to the grain. It can occur when metals are subjected to high temperatures (carbide precipitation) or due to defective welding. This lowers the material’s corrosion resistance, making it prone to damage when exposed to a corrosive agent.
Scale Your Manufacturing from Prototyping to Series
- Personal account manager
- Quality assurance
- Payment terms for companies
- On-time delivery by Fractory
Erosion Corrosion
Erosion corrosion is a type of corrosion that is caused by relative movement between the metal surface and a corrosive liquid. This movement results in mechanical abrasion which damages the surface and creates cavities.
The fluid usually flows at high velocities along the metal surface, dissolving the passive oxide layer and removing it as the fluid travels. Erosion corrosion occurs mostly inside metal tubes which are used to transfer corrosive liquids that slowly deteriorate the surface.
High Temperature Corrosion
High-temperature corrosion or hot corrosion occurs when a combination of high temperature (< 400°C) and atmospheric contaminants is present. A chemical attack occurs as the metals operating under these temperatures chemically react with the corrosive contaminants.
High-temperature corrosion is common in industrial environments where furnaces and gas turbines that have atmospheric contaminants and sulfuric gases are used. These corrosive contaminants might also leave ash deposits and molten salt while under operation. Cooling mechanisms and heat-resistant alloys are utilised to prevent high-temperature corrosion.
Stress Corrosion Cracking (SCC)

Stress corrosion cracking or corrosion fatigue is caused by applying tensile stress to the material while it is in a corrosive environment. Stress corrosion cracking can be especially prevalent when tensile stress is accompanied by temperature extremes. Metal expansion and contraction result from these temperature changes which weaken the structural integrity of the metal.
Initial signs of stress corrosion cracking are fine cracks on the metal surface. These cracks eventually develop over time, leading to structural failure. Stress corrosion can occur in manufacturing processes, such as machining and welding, but it is accelerated when exposed to a corrosive environment. Examples of this are stainless steels in a chloride environment and copper alloys in ammonia.
Microbial Corrosion
Microbial corrosion, also called microbiologically induced corrosion (MIC), is a type of corrosion caused by the presence and activities of microorganisms on metal surfaces. These microorganisms, which include bacteria, fungi, and algae, can accelerate the corrosion process in metals and alloys in various environments.
MIC is a significant concern in industries like oil and gas, marine, and wastewater management because it can lead to the rapid deterioration of materials. Some of these organisms are capable of consuming oil and excreting acids that can cause corrosion of vessels used for storing.
Microbiologically influenced corrosion can cause various types of damage, including pitting, crevice corrosion and stress corrosion cracking. To prevent this, the oil must be purified as much as possible to remove water content. Draining water at regular intervals from fuel tanks after purification is also necessary. Effective management of MIC also involves regular monitoring and using biocides to control microbial growth. Understanding the specific microbes and environmental conditions involved is key to developing targeted prevention and control strategies.
How to Prevent Corrosion
Preventing different corrosion types is crucial in maintaining the functionality and longevity of assemblies and equipment. Applying surface treatment, protective coatings or material finishing are great proactive measures to reduce the impact of corrosion. Using corrosion-resistant materials in a non-corrosive environment is a good corrosion prevention measure against most types of corrosion.

